Lithium Battery Electrolyte Crystallization vs Evaporation Guide
What is Lithium Battery Electrolyte?
Lithium battery electrolyte is a conductive medium that facilitates ion transport in lithium-ion batteries.
Its main components include:
- Lithium salts - Primary ionic conductors
- Organic solvents - Liquid carriers for ion movement
- Additives - Performance enhancers
The electrolyte serves two critical functions: ion conduction and film formation, both essential for battery performance and longevity.
Understanding Electrolyte Crystallization vs. Solvent Evaporation
The Common Misconception
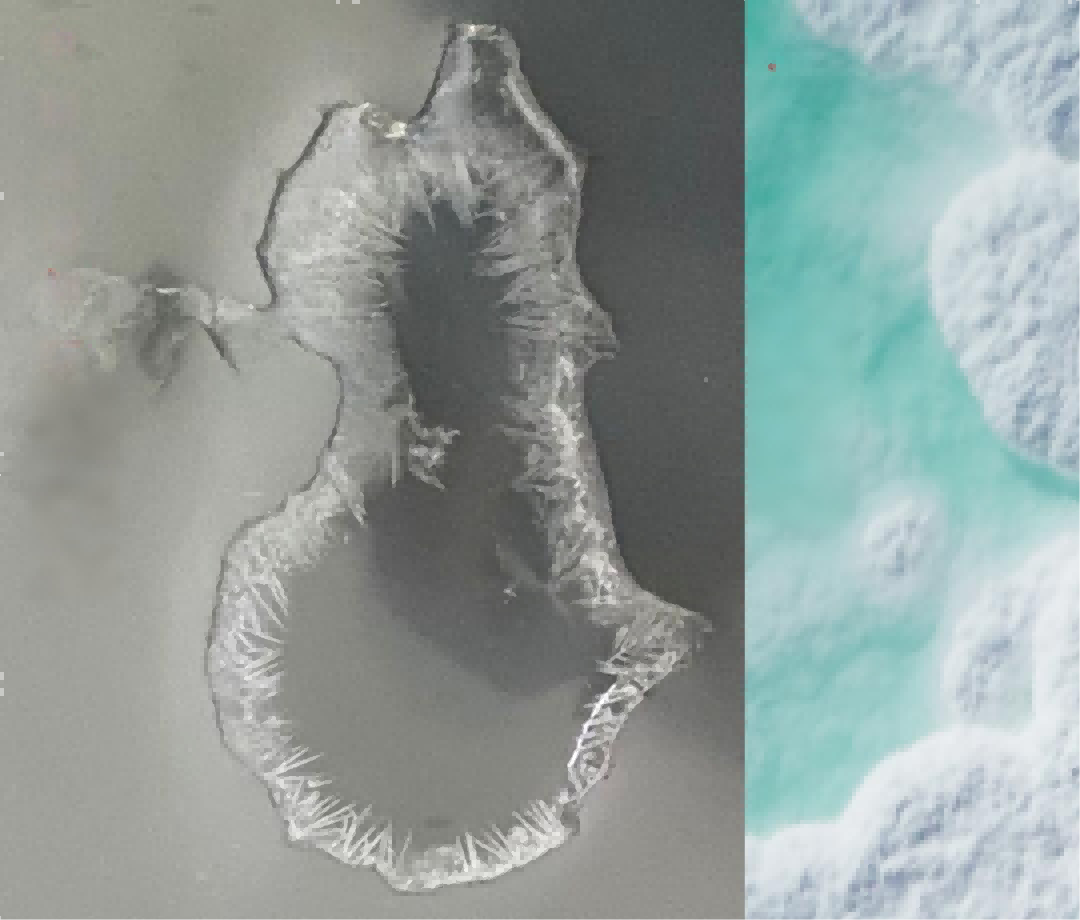
Many people mistakenly believe that when electrolyte solvent evaporates and dries, the electrolyte has crystallized. This is a major misconception. Crystallization and solvent evaporation to dryness are two completely distinct phenomena.
What is Electrolyte Crystallization?
Electrolyte crystallization occurs in two forms:
-
1. Solvent Crystallization
Solvent crystallization is the most common type in electrolytes. It occurs when:
- Temperature drops below the solvent's freezing point
- The liquid equilibrium of the solvent is disrupted
- Solvent molecules transition from disordered motion to ordered crystal structure
Key Point: Ethylene carbonate (EC) has a melting point of 36.4°C, making it prone to solidification at room temperature. The precipitated crystals are essentially EC.
Detection Method: EC can be detected using gas chromatography for verification. -
2. Lithium Salt Crystallization
Lithium salt crystallization follows this process: Dissolution → Supersaturation → Precipitation (primarily due to solubility limits).
Important Note: Industrial liquid lithium salts typically have a mass fraction around 30%, much higher than in electrolytes. Since most production uses linear carbonate solvents, lithium salt precipitation can generally be ruled out in standard conditions.
Detection Method: Lithium salts can be detected using ion chromatography.
What is Solvent Evaporation to Dryness?
Solvent evaporation to dryness is a simpler process where:
- The solvent completely evaporates
- Only the solute (lithium salt) remains behind
- A white residue mark is left on the surface
This is not crystallization - it's simply the physical removal of the liquid phase through evaporation.
Visual Comparison: Crystallization vs. Evaporation
| Phenomenon | Characteristics | State |
|---|---|---|
| Crystallization | Ordered crystal structures form while liquid remains. | Ordered crystal structures form while liquid remains. |
| Evaporation to Dryness | Complete liquid loss with residue formation. | Complete liquid loss with residue formation. |
Can You Measure Crystallization Time?
The Challenge of Measurement
Measuring crystallization time is extremely difficult, if not impossible, for several reasons:
- Subjective observation: Even the same person visually observing crystallization will get inconsistent results.
- Variable contact area: Two drops of equal mass may have different environmental contact areas.
- Volume effects: Larger volumes may never crystallize before evaporating completely.
- Time variability: After 2-3 hours, evaporation may occur instead of crystallization.
Why Companies Don't Measure It
No company invests significant effort to determine whether crystallization occurs at 5 minutes, 10 minutes, or 1 hour because:
- The question lacks practical significance.
- Meeting production and process requirements is sufficient.
- Qualitative assessment is challenging enough without attempting quantification.
How to Prevent or Improve Electrolyte Crystallization
There are three main approaches to addressing crystallization issues:
-
1. Environmental Control
Temperature and Humidity Management:
- Maintain temperatures above solvent freezing points.
- Avoid excessively low humidity conditions.
- Control ambient conditions during production and storage.
-
2. Process Optimization
Key Process Variables:
- Interval time: Adjust timing between process steps.
- Nozzle design: Optimize nozzle shape and dimensions.
- Dispensing method: Control droplet size and placement.
-
3. Electrolyte Formulation Adjustment
Formulation Strategies:
- Reduce EC Content: EC's 36.4°C melting point makes it prone to room-temperature solidification. Blending EC with other carbonate solvents lowers the system's melting point.
- Increase Lithium Salt Concentration: Higher lithium salt concentration inhibits crystallization and creates a more stable solution at lower temperatures.
- Solvent Selection: Use linear carbonate solvents to improve low-temperature performance.
Practical Considerations for Battery Manufacturers
Quality Control Approach
Rather than attempting precise crystallization time measurements, focus on:
- Environmental adjustments: Fine-tune temperature and humidity.
- Formulation tweaks: Make minor composition changes.
- Equipment modifications: Change nozzle specifications as needed.
- Process validation: Ensure production requirements are consistently met.
Common Questions Addressed
Q: How long does it take for electrolyte to evaporate?
This question is difficult to answer meaningfully because evaporation rate depends on numerous variables: surface area, temperature, humidity, and airflow all play roles. The question lacks practical application value.
Q: How can I tell if crystallization has occurred?
- Visible crystal formation while liquid remains present
- Ordered structures rather than simple residue
- Reversibility when temperature increases (crystals melt back to liquid)
Conclusion
Understanding the difference between electrolyte crystallization and solvent evaporation is crucial for lithium battery manufacturing and quality control. While crystallization involves ordered molecular structure formation due to temperature changes, evaporation simply removes the liquid phase entirely.
For optimal battery performance, focus on the three key improvement areas: environmental control, process optimization, and formulation adjustment. Rather than attempting to measure crystallization time precisely, prioritize meeting production requirements through practical adjustments.

