This analysis explores how lithium salt concentration and temperature jointly affect Li-ion transport in LiPF6/EC electrolytes. Using a wide 0.2–5M concentration range and temperatures from -40°C to 25°C (233–298 K), the findings provide practical guidance for designing lithium-ion batteries capable of delivering strong performance in cold environments.
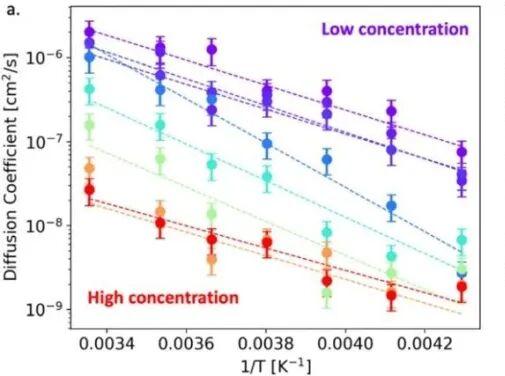
Across all electrolyte formulations, the diffusion coefficient of Li-ions decreases as temperature decreases. However, the magnitude of this drop is strongly influenced by the electrolyte concentration and does not follow a simple linear trend.
Using Arrhenius fitting, electrolytes with higher pre-exponential factors and lower activation energies demonstrate significantly improved ion mobility at low temperatures. These systems exhibit reduced internal resistance and maintain better performance under cold conditions.
Why Concentration Is the Dominant Variable at Low Temperatures
Low Salt Concentration (0.2–0.5M): The Clear Low-Temperature Advantage
Electrolytes in the 0.2–0.5M range consistently deliver the highest relative Li-ion mobility at sub-zero temperatures. Lower viscosity and minimal ion clustering create a larger population of fast-moving solvated Li-ions.
High Concentration Limitations
As concentration increases toward 5M, both the pre-exponential factor and activation energy decrease exponentially. This indicates severe restrictions on Li-ion mobility and significantly higher diffusion resistance.
The Trade-Off at 1M
The widely used 1M LiPF6 formulation shows the highest diffusion at room temperature. Yet, this same concentration exhibits pronounced performance decay at low temperatures due to increased viscosity and stronger ion interactions.
Microscopic Mechanisms: How Solvation and Ion Structures Control Li-Ion Transport
1. Concentration-Driven Structural Changes
Increasing LiPF6 concentration alters the solvation environment of Li-ions:
- Solvent Coordination Drops Sharply: EC molecules in the first solvation shell decrease from ~5 (at 0.2M) to ~2 (at 5M).
- Ion Pairing Intensifies: PF6⁻ anions increasingly replace solvent molecules around Li-ions.
- Cluster Formation: At 5M, the number of PF6⁻ coordinating each Li-ion exceeds that of EC, resulting in extensive ion pairing and aggregation.
This clustering immobilises a significant proportion of Li-ions, reducing the fraction of highly mobile “free” solvated ions. The result is a measurable decline in diffusion and ionic conductivity.
2. Temperature Has a Secondary Structural Impact
At a fixed concentration (e.g., 1M), cooling the electrolyte from 25°C to -40°C produces only minor changes:
- EC coordination decreases slightly from 5.4 to 4.7.
- Li⁺–PF6⁻ ion pairing remains nearly unchanged.
These small variations arise mainly from reduced thermal motion rather than fundamental shifts in electrolyte structure. Thus, temperature influences ion mobility primarily through viscosity and kinetic effects—not structural reorganisation.
Key Design Insights for Low-Temperature Lithium-Ion Batteries
This study highlights a critical fact: LiPF6/EC electrolyte structure is primarily governed by concentration, not temperature. For real-world battery design, this means:
- Adjusting salt concentration is far more effective than thermal management when optimising for cold-weather operation.
- Reducing concentration within a controlled range can minimise ion clustering and maximise the population of fast-moving solvated Li-ions.
- High-concentration electrolytes may offer benefits at high temperatures but become inherently diffusion-limited in sub-zero environments.
To improve low-temperature lithium-ion battery performance, the most impactful lever is precise electrolyte concentration tuning, which directly controls ion aggregation, transport pathways, and the overall electrochemical behaviour of the system.

