Enhancing Fast-Charging Performance of Graphite Anodes in Lithium-Ion Batteries | Surface Coating Strategies and Materials
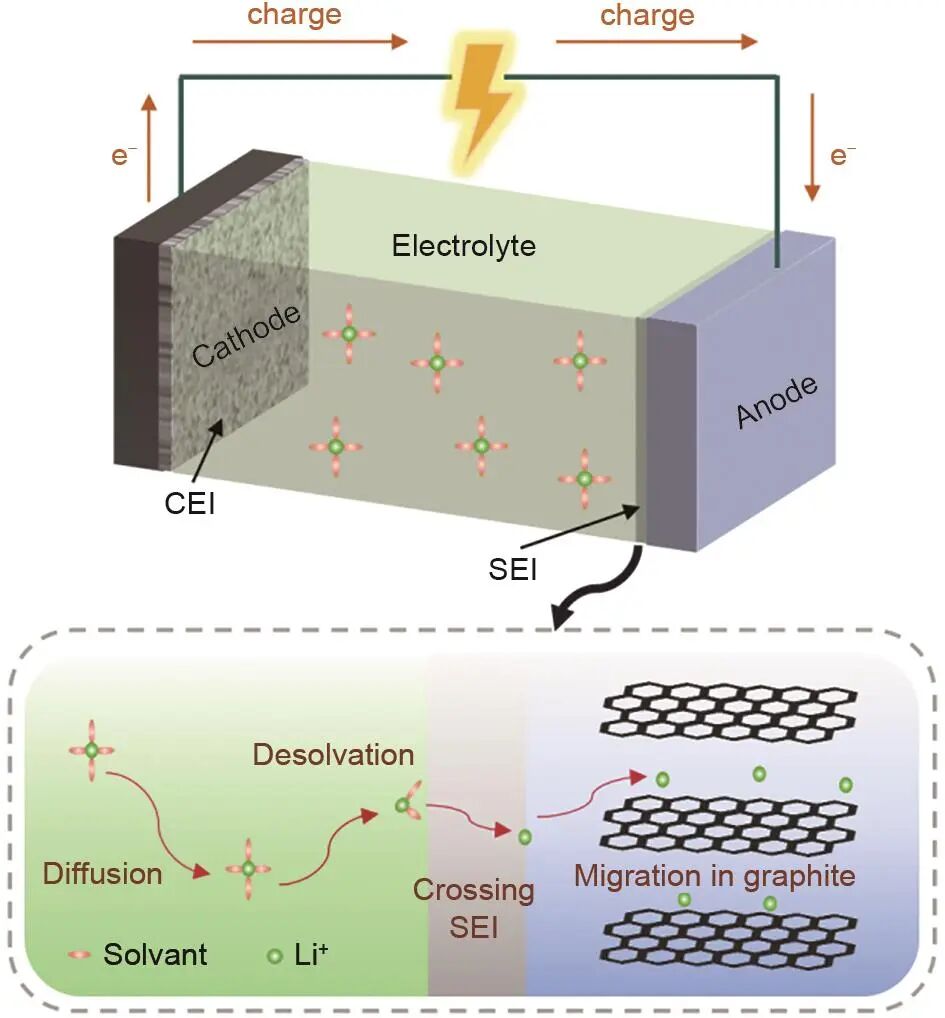
Figure 1: Schematic diagram of the working principle and charging process of LIBs
Graphite Anode: Mechanisms and Rate-Limiting Steps
Graphite remains the most widely used anode material in lithium-ion batteries (LIBs) due to its high energy density, low operating voltage, excellent electrical conductivity, abundant availability, and low cost. Owing to these advantages, graphite will continue to serve as the dominant anode material for LIBs for the foreseeable future.
The Charging Process (Key Steps)
- Lithium ions (Li+) are deintercalated from the cathode and diffuse through the electrolyte.
- Li+ becomes solvated by electrolyte molecules.
- The solvated Li+ migrates across the separator to reach the surface of the graphite anode.
- At the solid electrolyte interphase (SEI), Li+ undergoes desolvation and diffuses through the SEI layer.
- Finally, Li+ diffuses within the graphite particle bulk.
The diffusion rate of Li+ at each stage directly impacts the fast-charging performance of LIBs. Among these processes, Li+ desolvation at the SEI surface and diffusion inside the graphite lattice are especially critical.
Fast-Charging Bottlenecks
The layered structure of graphite requires Li+ to intercalate from the edge planes of particles and gradually diffuse inward, resulting in a long diffusion path. Moreover, the narrow interlayer spacing (0.335 nm) of graphite limits ion transport, thereby constraining fast-charging capability. Under high-rate charge/discharge conditions, a steep Li+ concentration gradient forms at the electrode–electrolyte interface, leading to severe concentration polarization. This can cause lithium plating on the graphite surface, forming lithium dendrites that degrade capacity, shorten cycle life, and pose serious safety risks.
Surface Coating: A Promising Solution
Surface coating has emerged as one of the most effective strategies to mitigate these challenges by reducing surface concentration polarization and enhancing Li+ transport kinetics.
Primary Functions of Coating
- Shielding active sites on the graphite surface to suppress irreversible side reactions;
- Constructing a stable, Li+-conductive SEI layer that facilitates fast ion transfer;
- Lowering the Li+ diffusion energy barrier through interfacial chemical bonding, thereby accelerating Li+ transport within the solid phase.
A wide range of coating materials has been explored to optimize graphite anode performance, including amorphous carbon, metal oxides, polymers, and inorganic compounds. These coatings promote the desolvation of solvated Li+ ions, suppress lithium plating, and alleviate interfacial instability during high-rate operation.
Among them, amorphous carbon coatings are particularly effective. Due to their larger interlayer spacing compared with crystalline graphite, they significantly improve Li+ diffusion kinetics. In addition, the higher lithiation potential of amorphous carbon helps suppress lithium deposition, thereby enhancing the high-rate charge–discharge capability and overall fast-charging performance of graphite anodes.
Example Coating Structures and Performance
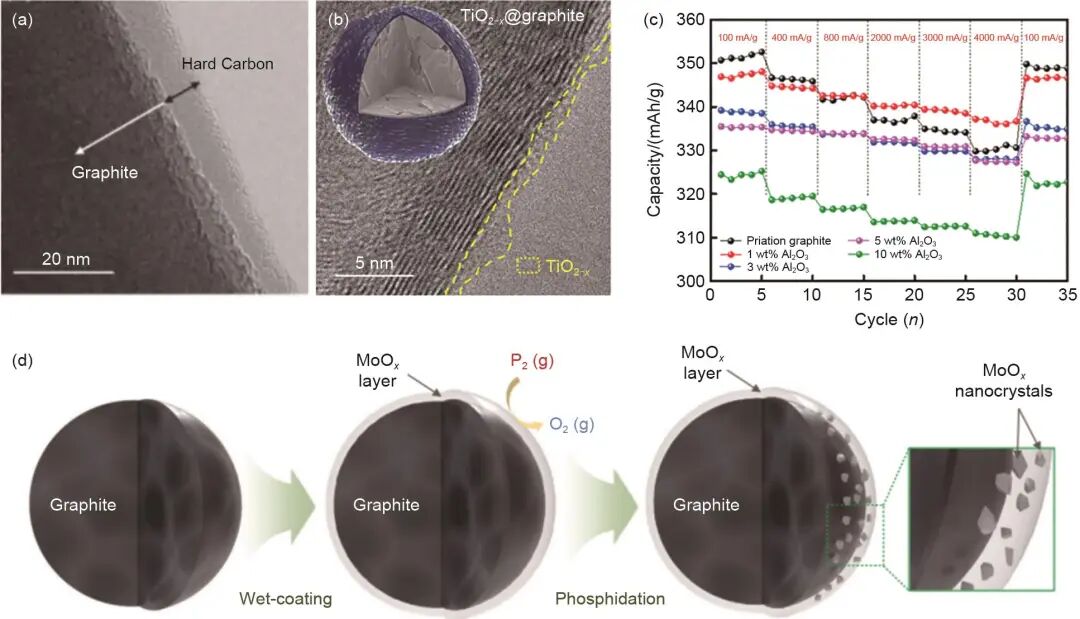
Figure 2: Representative structures for fast-charging applications
Representative examples of graphite surface coating structures for fast-charging applications include:
- (a) Hard carbon–coated graphite: schematic of the microstructural morphology.
- (b) TiO2-x@graphite core–shell structure: the TiO2-x layer effectively reduces interfacial resistance between the electrode and the electrolyte.
- (c) Al2O3-coated graphite: varying coating thickness influences rate capability — for instance, graphite with 1 wt% Al2O3 coating achieves a reversible specific capacity of ~337.1 mAh/g at 100 mA/g.
- (d) MoOx–MoPx/graphite composite anode: the combination of MoOx and nanoscale MoPx effectively suppresses lithium plating during fast charging.
Summary: The Path to High-Rate LIBs
Surface coating modification provides a powerful route to overcome the intrinsic limitations of graphite anodes by improving interfacial ion transport, reducing polarization, and enhancing rate performance. Rational design of coating materials—such as amorphous carbon layers, metal oxide shells, or hybrid composite coatings—offers a pathway to achieve high-performance, fast-charging, and safe graphite-based lithium-ion batteries.

